Why in Discussion ?
- Recently, the deepest gas hydrate–associated “cold seep” discovered so far has been recorded on the seafloor off western Greenland.
- At this site, natural gas—mainly methane—was observed escaping as bubbles from ice-like hydrate cages.
- The area was also found to be rich in biodiversity, bringing renewed attention to gas hydrates from the perspectives of energy security, climate change, and deep-sea ecology.
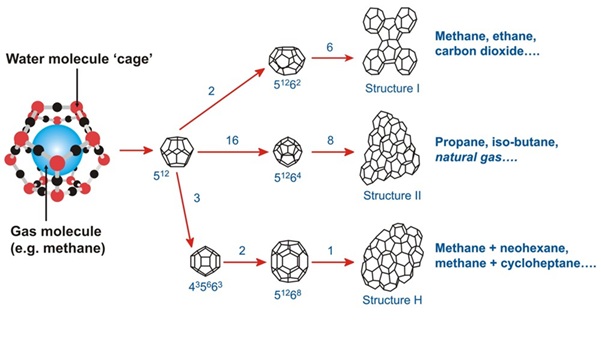
What Are Gas Hydrates ?
- Gas hydrates are ice-like crystalline solid compounds that form when low-density gases such as:Methane (CH₄), Ethane (C₂H₆), or Carbon dioxide (CO₂) become physically
- trapped within water molecules under conditions of:Low temperature, and Moderate to high pressure.
- Importantly, no chemical bond is formed between gas and water; the gas is only physically enclosed.
Structure and Classification (Clathrates)
- Gas hydrates belong to a class of compounds known as clathrates.
- Structural features:
- Water molecules arrange themselves into a three-dimensional cage-like lattice.
- Gas molecules occupy the cavities inside these cages.
- Key characteristic:
- Absence of chemical bonding, which makes hydrates relatively unstable if temperature or pressure changes.
- Important fact:-Since most gas hydrates are methane-based, the terms “gas hydrate” and “methane hydrate” are often used interchangeably.
Conditions Required for Formation
Gas hydrates form only when the following conditions coexist:
- Low temperature
- Moderate to high pressure
- Availability of water and gas
Therefore, they are typically found in:
- Deep-sea sediments, and
- Permafrost regions (Arctic and sub-Arctic), both within and beneath frozen ground.
Distribution on Earth
Gas hydrates are mainly distributed in:
- Continental shelves and slopes
- Deep ocean floor sediments
- Arctic permafrost zones
Scientists also hypothesize that under suitable conditions, gas hydrates may exist on other planets or icy moons as well.
Stability and Decomposition
- Gas hydrates remain stable only within a narrow pressure–temperature window.
- When:Temperature increases, or Pressure decreases, the hydrate breaks down as follows:Gas Hydrate → Water + Gas
- This decomposition can release large quantities of methane into the surrounding environment.
Significance of Gas Hydrates
(a) As an Energy Resource
- Estimates suggest that the carbon stored in gas hydrates may be:
- Nearly twice the combined carbon content of coal + oil + conventional natural gas.
- Hence, gas hydrates are viewed as a potential future energy source, though technological and environmental challenges remain.
(b) Link with Climate Change
- Methane is a highly potent greenhouse gas, far stronger than CO₂ over short timescales.
- Large-scale destabilization of gas hydrates could:
- Release massive amounts of methane,
- Accelerate global warming, and
- Act as a climate feedback mechanism.
(c) Geophysical Hazards
- Sudden methane release from seafloor hydrates may trigger:
- Submarine landslides, and
- Potentially tsunami-generating events in extreme cases.
(d) Biodiversity and Chemosynthesis
- Cold seep ecosystems associated with gas hydrates host:
- Unique and specialized biological communities.
- These organisms rely on chemosynthesis, not sunlight, to survive:
- They derive energy and carbon from hydrocarbons or hydrogen sulfide.
- This makes such ecosystems biologically and evolutionarily significant.
Significance of the Greenland Cold Seep Discovery
- Represents the deepest known gas hydrate–related cold seep discovered to date.
- Demonstrates that:
- Gas hydrates can remain stable even at extreme ocean depths.
- Rich and complex ecosystems can develop in such environments.
- The discovery provides valuable insights into the interconnected nature of energy resources, climate processes, and deep-sea biodiversity.



